BiomX: A Major Catalyst on the Horizon
Taking a Look at BiomX (NYSE: PHGE), Weekly Activity, & Portfolio Update
What Does BiomX Do?
BiomX (PHGE) is a pioneering biotechnology firm, still in the pre-revenue stage, utilizing innovative bacteriophage (phage) therapy to combat specific diseases, specifically targeting Atopic Dermatitis (BX005) and Cystic Fibrosis (BX004) in their development pipeline. They achieve this through their proprietary platforms, BOLT and XMarker, which will be elaborated on shortly. To streamline their focus and allocate resources effectively, BiomX has recently suspended two of their programs, channeling their efforts primarily towards the promising candidates, BX005 and, more intensively, CF BX004. The company stands as a unique entity in advancing phage therapy, navigating a field marked by numerous setbacks, being at the leading edge of drug development in this domain. The company meticulously crafts and standardizes indispensable tools needed for the discovery, validation, engineering, testing, and manufacturing of phage therapies, maintaining a methodical approach to ensure precision and reliability throughout the developmental process. They are committed to forging paths in phage therapy, aiming to address and resolve critical medical conditions with groundbreaking solutions.
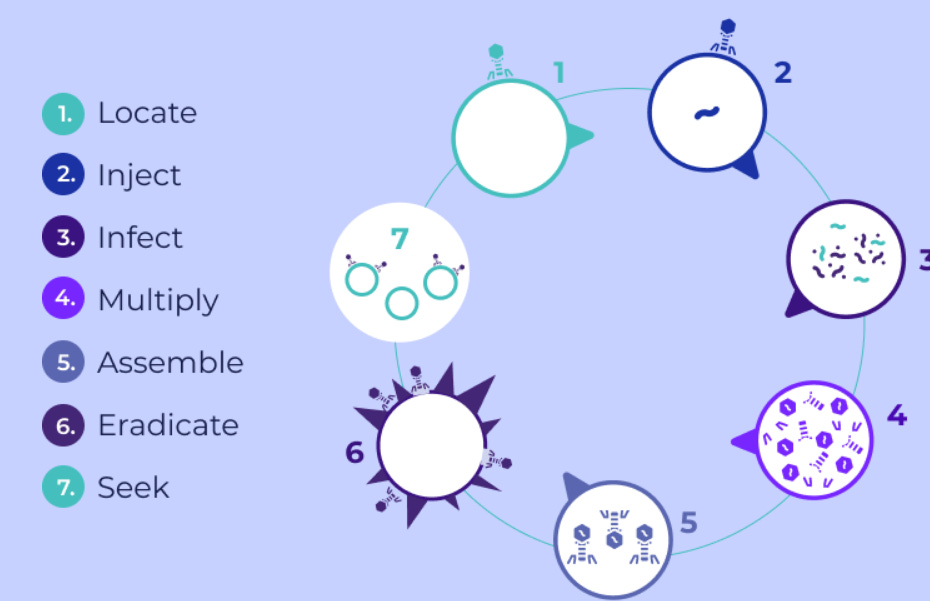
The people up at BiomX, are using a new way of treating diseases, using bacteriophages. Bacteriophages, also known as “phages” or “phage therapy”, are viruses specialized in invading bacteria, having no impact on mammalian cells, thus considered inert to them. These phages are the natural antagonists of bacteria and can be found in any environment where bacteria thrive. They exhibit specificity to distinct bacterial species or strains, enabling them to attack and annihilate their targets while leaving the surrounding, possibly beneficial, bacteria unharmed and intact. This ensures a targeted approach to bacterial eradication without disturbing the ecological balance of the microbial community.
BiomX is creating these phage concoctions designed to identify and eradicate these harmful bacteria. The concoctions, refined through sophisticated algorithms and validated experimentally both in-vitro and in-vivo, consist of several natural and/or modified phages. These selected phages boast of synergistic functionalities, such as an extensive range of bacterial host targeting and the capability to circumvent the development of bacterial resistance, among other distinguished features. The meticulous optimization ensures the efficacy of these phage cocktails in targeting and neutralizing pathogenic bacteria.
In much simpler terms, BiomX is taking natural viruses that are not harmful to humans, then reprogramming them to identify and eradicate specific diseases. Here is a visual of how a phage operates in the human body (click on the link):
The integrated platform it utilizes consists of two pivotal technology platforms: XMarker and BOLT. XMarker, a cutting-edge metagenomics platform, is designed to pinpoint bacterial biomarkers linked to infectious diseases. This enables BiomX to comb through the gut microbiomes of individuals with inflammatory bowel disease (IBD) to discern patterns in bacterial DNA that elevate disease severity and progression, and subsequently create phage therapies targeting specific strains of detrimental bacteria.
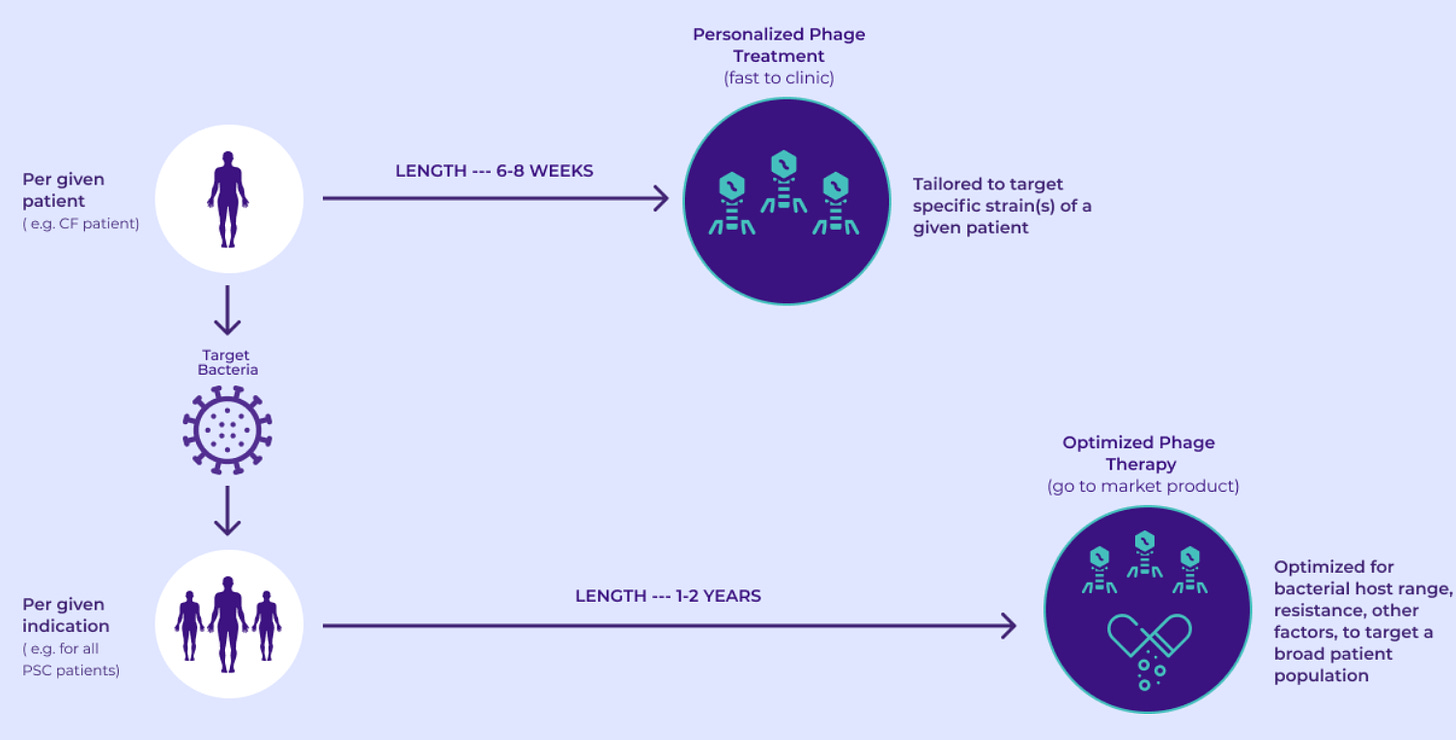
BOLT, standing for "BacteriOphage Lead to Treatment," plays a crucial role in formulating phage therapy drug candidates. It enables BiomX to create both personalized and optimized treatment solutions, targeting individual bacterial strains or a spectrum of common strains respectively. Additionally, it presents the possibility of phages administering therapeutic drug payloads, offering another avenue of treatment.
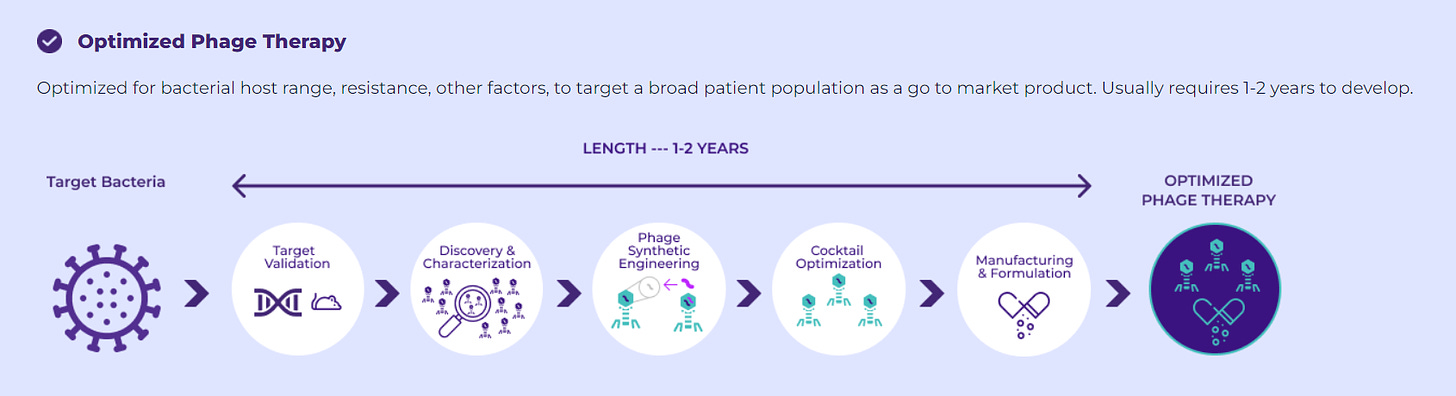
The BOLT platform, standing for “BacteriOphage Lead to Treatment”, integrates proprietary computational tools, automated screening, synthetic engineering capabilities, and diverse validation assays, all orchestrated to formulate natural or engineered phage cocktails pinpointing specific pathogenic bacteria. We employ this platform to cultivate phage cocktails, navigating them through two distinctive development trajectories as delineated above. This systematic approach ensures precision and effectiveness in targeting and eradicating harmful bacterial strains while preserving beneficial ones.
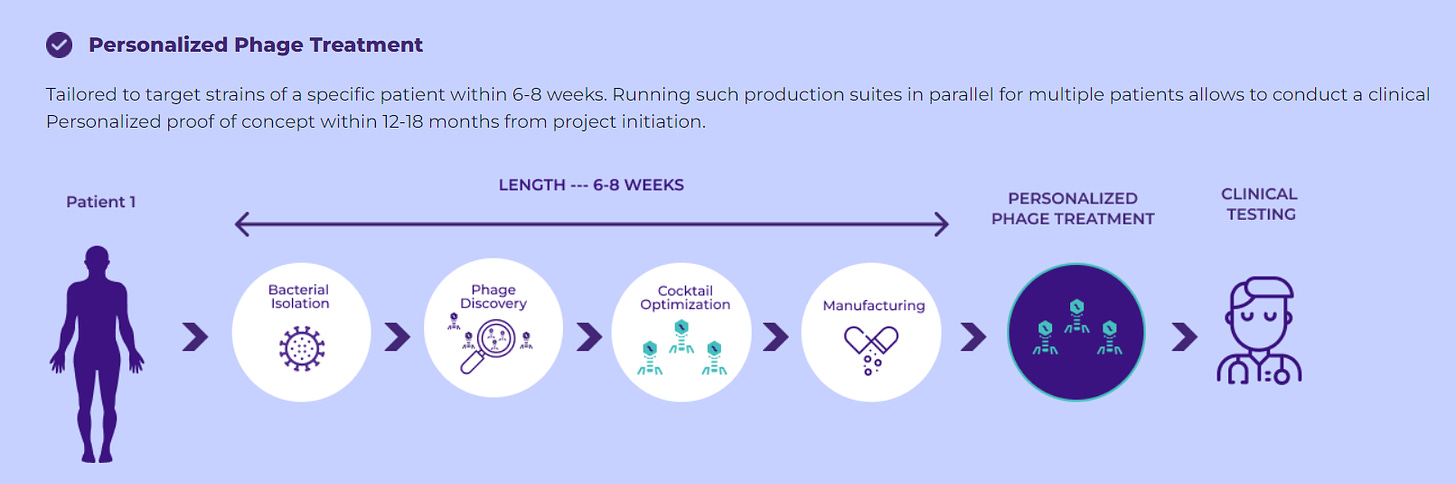
Utilizing these technologies and the inherent benefits of phages, BiomX can substantially expedite the drug development process. Bolt-designed phage therapy candidates can advance from discovery to clinical development in under eight weeks, with the potential to yield phase 1 data in under 18 months, subject to regulatory approval. This is a stark contrast to traditional drug development timelines, which can span up to five years from discovery to the conclusion of phase 1 clinical trials (massive advantage).
Several industry stalwarts back both technology platforms. XMarker, integral for biomarker discovery in IBD, is employed by Janssen, a Johnson & Johnson subsidiary, and Boehringer Ingelheim. Concurrently, the venture capital branches of Takeda, OrbiMed, and Johnson & Johnson have invested in BiomX in its private fundraising rounds prior to its public listing.
While BiomX’s rigorous and comprehensive approach to developing a seamless platform doesn’t assure success, the pursuit of a phage therapy platform would likely be futile without a similar commitment to standardization and meticulous development.
So, What’s The Big Deal?
Keep reading with a 7-day free trial
Subscribe to Steve Wagner | Invest to keep reading this post and get 7 days of free access to the full post archives.